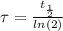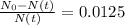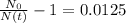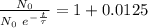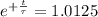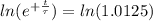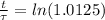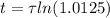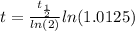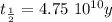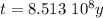The ages of rocks that contain fossils can be determined using the isotope 87rb. this isotope of rubidium undergoes beta decay with a half‑life of 4.75×1010y . ancient samples contain a ratio of 87sr to rb87 of 0.0125. given that 87sr is a stable product of the beta decay of 87rb, and assuming there was originally no 87sr present in the rocks, calculate the age of the rock sample. assume that the decay rate is constant over the relatively short lifetime of the rock compared to the half-life of 87rb.

Answers: 1


Another question on Physics


Physics, 22.06.2019 17:50
Which of the following best describes internal energy? a. the difference between the kinetic and potential energies of the particles in a system b. the sum of the kinetic and potential energies of the particles in a system c. the sum of the kinetic and thermal energies of the particles in a system d. the difference between the kinetic and thermal energies of the particles in a system
Answers: 2

Physics, 23.06.2019 02:30
Five renewable energy resources are wind, sunlight, moving water and geothermal energy. question 2 options: true false
Answers: 2

You know the right answer?
The ages of rocks that contain fossils can be determined using the isotope 87rb. this isotope of rub...
Questions


Mathematics, 07.06.2020 02:00





English, 07.06.2020 02:00


Physics, 07.06.2020 02:00


Mathematics, 07.06.2020 02:00

History, 07.06.2020 02:00






History, 07.06.2020 02:00


Mathematics, 07.06.2020 02:00

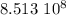 years
years 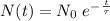
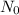 is the initial quantity of the material, and
is the initial quantity of the material, and  is the mean lifetime of the material.
is the mean lifetime of the material.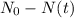
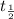 ) by the relationship
) by the relationship