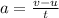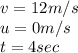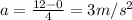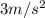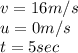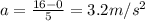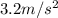Answers: 1


Another question on Physics

Physics, 22.06.2019 12:30
An ice-making machine inside a refrigerator operates in a carnot cycle. it takes heat from liquid water at 0.0 degrees celsius and rejects heat to a room at a temperature of 19.2 degrees celsius. suppose that liquid water with a mass of 76.3kg at 0.0 degrees celsius is converted to ice at the same temperature. take the heat of fusion for water to be l_f = 3.34*10^5 j/kg.how much energy e must be supplied to the device? express your answer in joules.
Answers: 1

Physics, 22.06.2019 17:40
The weights of bags filled by a machine are normally distributed with a standard deviation of 0.05 kilograms and a mean that can be set by the operator. at what level should the mean weight be set if it required that only 1% of the bags weigh less than 9.5 kilograms? round the answer to 2 decimal places.
Answers: 1

Physics, 22.06.2019 18:40
Which body is in equilibrium? (1) a satellite orbiting earth in a circular orbit (2) a ball falling freely toward the surface of earth (3) a car moving with a constant speed along a straight, level road (4) a projectile at the highest point in its trajectory
Answers: 2

Physics, 23.06.2019 00:30
Which of the following statements are true for an isothermal process? check all that apply. - during an isothermal process, the work done by the gas equals the heat added to the gas. - during an isothermal process, the internal energy of the system changes. - an isothermal process is carried out at constant temperature. - an isothermal process is carried out at constant pressure. - an isothermal process is carried out at constant volume.
Answers: 1
You know the right answer?
Riding his bike, dewayne can start from rest and get going at 12m/s in 4 seconds. beth can get going...
Questions








Computers and Technology, 10.12.2019 06:31



Computers and Technology, 10.12.2019 06:31