
Physics, 24.09.2019 05:00 lexybellx3
Suppose 16.0 g of oxygen (o2) is heated at constant atmospheric pressure from 26.4°c to 111°c. (a) how many moles of oxygen are present? (take the molar mass of oxygen to be 32.0 g/mol) (b) how much energy is transferred to the oxygen as heat? (the molecules rotate but do not oscillate.) (c) what fraction of the heat is used to raise the internal energy of the oxygen?

Answers: 1


Another question on Physics



Physics, 22.06.2019 14:30
When the displacement of a mass on a spring is 12a the half of the amplitude, what fraction of the mechanical energy is kinetic energy? at what displacement, as a fraction of a, is the mechanical energy half kinetic and half potential?
Answers: 3

Physics, 22.06.2019 18:20
Awave with a frequency of 500 hz is traveling at a speed of 100 m/s.what is the wavelength?
Answers: 1
You know the right answer?
Suppose 16.0 g of oxygen (o2) is heated at constant atmospheric pressure from 26.4°c to 111°c. (a) h...
Questions
















Biology, 30.05.2020 17:00




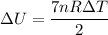 ----1
----1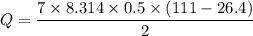
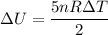 --------2
--------2

