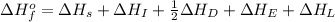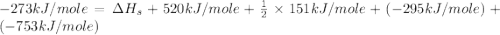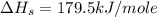Physics, 07.10.2019 18:10 dorafacegirl
Consider the following: li(s) (g) n lii(s) 222 kj. lii(s) has a lattice energy of kj/mol. the ionization energy of li(g) is 520. kj/mol, the bond en- ergy of i2(g) is 151 kj/mol, and the electron affinity of i(g) is kj/mol. use these data to determine the heat of sublimation of li(s

Answers: 1


Another question on Physics

Physics, 22.06.2019 02:40
If the wheels lock when braking suddenly the vehicle: a: loses traction b: lose steering wheel ability c: gain speed slightly d: gain steering ability
Answers: 1

Physics, 22.06.2019 05:30
What are similarities and differences of reflection, refraction, diffraction and absorption?
Answers: 1

Physics, 22.06.2019 16:00
Two balls, each with a mass of 0.5 kg, collide on a pool table. is the law of conservation of momentum satisfied in the collision? explain why or why not
Answers: 1

Physics, 22.06.2019 17:40
A15.75-g piece of iron absorbs 1086.75 joules of heat energy, and its temperature changes from 25°c to 175°c. what is the specific heat capacity of iron?
Answers: 1
You know the right answer?
Consider the following: li(s) (g) n lii(s) 222 kj. lii(s) has a lattice energy of kj/mol. the...
Questions


Mathematics, 30.01.2020 15:00

Computers and Technology, 30.01.2020 15:00

Mathematics, 30.01.2020 15:00

Mathematics, 30.01.2020 15:00

Arts, 30.01.2020 15:00


Social Studies, 30.01.2020 15:00

SAT, 30.01.2020 15:00

Mathematics, 30.01.2020 15:00



Mathematics, 30.01.2020 15:00


Biology, 30.01.2020 15:00



Mathematics, 30.01.2020 15:00

Mathematics, 30.01.2020 15:00

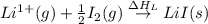
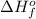 = enthalpy of formation of lithium iodide
= enthalpy of formation of lithium iodide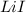 :
: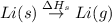
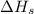 = sublimation energy of lithium
= sublimation energy of lithium 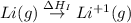
 = ionization energy of lithium
= ionization energy of lithium 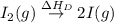
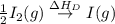
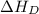 = dissociation energy of iodine
= dissociation energy of iodine 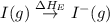
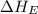 = electron affinity energy of iodine
= electron affinity energy of iodine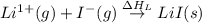
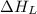 = lattice energy of lithium iodide
= lattice energy of lithium iodide