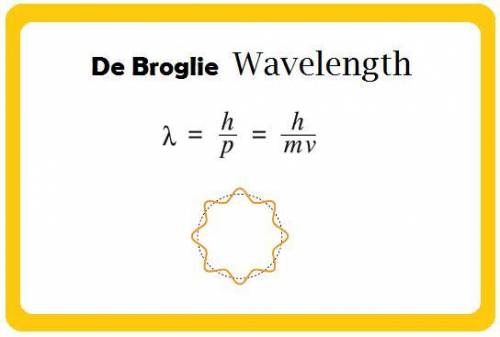
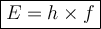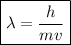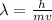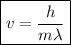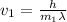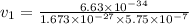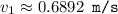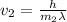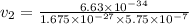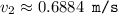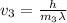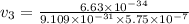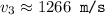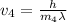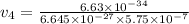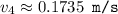Physics, 19.10.2019 00:00 JaquoiaDean9823
What would the speed of each particle be if it had the same wavelength as a photon of yellow light =575.0 nm)? proton (mass=1.673 x 10^−24 g) neutron (mass = 1.675 x 10–24 g)electron (mass = 9.109 x 10^–28 g)alpha particle (mass = 6.645 x 10^–24 g)

Answers: 3


Another question on Physics


Physics, 22.06.2019 17:00
If a negatively charged particle is placed at rest in an electric potential field that increases in the positive x-direction, what will the particle do? a. accelerate in the positive x-direction b. remain at rest c. accelerate in the negative x-direction
Answers: 3

Physics, 22.06.2019 17:20
Select all the correct answers.which two statements are true? a moving magnetic field creates an electric field.a constant magnetic field creates an electric field.a constant electric field creates a magnetic field.a moving electric field creates a magnetic field.resetnext
Answers: 1

Physics, 22.06.2019 18:00
Air enters a gas turbine with two stages of compression and two stages of expansion at 100 kpa and 17°c. this system uses a regenerator as well as reheating and intercooling – the intercooler returns the air to the inlet temperature. the pressure ratio across each compressor is 4 ; 300 kj/kg of heat are added to the air in each combustion chamber; and the regenerator operates perfectly while increasing the temperature of the cold air by 20°c. determine the system’s thermal efficiency. assume isentropic operations for all compressor and the turbine stages and use constant specific heats at room temperature. (0.378)
Answers: 3
You know the right answer?
What would the speed of each particle be if it had the same wavelength as a photon of yellow light =...
Questions

Social Studies, 24.12.2019 17:31


English, 24.12.2019 17:31



Arts, 24.12.2019 17:31

Chemistry, 24.12.2019 17:31



Advanced Placement (AP), 24.12.2019 17:31

History, 24.12.2019 17:31

Chemistry, 24.12.2019 17:31


Biology, 24.12.2019 17:31


English, 24.12.2019 17:31


Social Studies, 24.12.2019 17:31


Mathematics, 24.12.2019 17:31

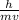
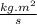
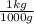 =1.673×10⁻²⁷ kg
=1.673×10⁻²⁷ kg