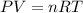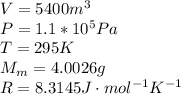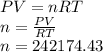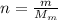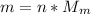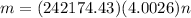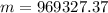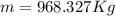Physics, 05.12.2019 23:31 kenishawilkinsoy4mgw
Agoodyear blimp typically contains 5400 m³ of helium (he) at an absolute pressure of 1.10 × 10 5 1.10 × 10 5 pa. the temperature of the helium is 295 k. what is the mass (in kg) of the helium in the blimp? (the molar mass of helium is 4.0026 g)

Answers: 3


Another question on Physics

Physics, 22.06.2019 07:20
Use the information presented in the graph to answer the questions. which segments show acceleration? which segment indicates that the object is slowing down? what is the velocity of segment b? what is the acceleration of segment b?
Answers: 3

Physics, 22.06.2019 11:30
Two 1.20-m nonconducting wires meet at a right angle. one segment carries + 2.50 µc of charge distributed uniformly along its length, and the other carries - 2.50 µc distributed uniformly along it, as shown in fig. 21.50. ( a. find the magnitude and direction of the electric field these wires produce at point p, which is 60.0 cm from each wire. ( b. if an electron is released at p, what are the magnitude and direction of the net force that these wires exert on it?
Answers: 3

Physics, 22.06.2019 16:30
Atank has a shape of a cone with a radius at the top of 2 m and a height of 5 m. the tank also has a 1 m spout at the top of the tank. the tank is filled with water up to a height of 2 m. find the work needed to pump all the water out the top of the spout. (use 9.8 m/s2 for g and the fact that the density of water is 1000 kg/m3.)
Answers: 1

Physics, 22.06.2019 16:40
Panel bc in fig. p2.76 is semi-circular, with the 3 meter radius and horizontal straight edge through point b. compute (a) the hydrostatic force of the water on the panel, (b) its center of pressure, and (c) the moment of this force about point b. assume atmospheric pressure on the dry side of the panel
Answers: 3
You know the right answer?
Agoodyear blimp typically contains 5400 m³ of helium (he) at an absolute pressure of 1.10 × 10 5 1.1...
Questions

Mathematics, 17.04.2020 22:42

Mathematics, 17.04.2020 22:42

Geography, 17.04.2020 22:42

Biology, 17.04.2020 22:42




Social Studies, 17.04.2020 22:42

English, 17.04.2020 22:42






Advanced Placement (AP), 17.04.2020 22:42

English, 17.04.2020 22:42



Advanced Placement (AP), 17.04.2020 22:42