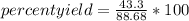The percent yield for the reaction is 48.82%
Explanation:
In order to determine the percentage of reaction yield, it is first necessary to determine which is the limiting reagent. The limiting reagent is that reagent that is consumed first in a chemical reaction, determining the amount of product obtained. The reaction depends on the limiting reagent because the other reagents will not react when one has been consumed.
For determine which is the limiting reagent, you know that:
P₄=31 g/mol (1 P) *4= 124 g/molO₂= 16 g/mol (1 O) *2= 32 g/molP₄O₆=31 g/mol (1 P) *4 + 16 g/mol (1 O) *6= 220 g/mol
By reaction stoichiometry of the reaction (that is, the relationship between the amount of reagents and products in a chemical reaction), reacts 1 mole of phosphorus P₄ and 3 moles of oxygen O₂, and produce 1 mol of P₄O₆ then:
P₄= 124 g/mol *1 mol= 124 gO₂= 32 g/mol *3 mol= 96 gP₄O₆= 220 g/mol*1 mol= 220 g
The rule of three or is a way of solving problems of proportionality between three known values and an unknown value, establishing a relationship of proportionality between all of them. That is, what is intended with it is to find the fourth term of a proportion knowing the other three. Remember that proportionality is a constant relationship or ratio between different magnitudes.
If the relationship between the magnitudes is direct, that is, when one magnitude increases, so does the other (or when one magnitude decreases, so does the other) , the direct rule of three must be applied. To solve a direct rule of three, the following formula must be followed:
a ⇒ b
c ⇒ x
Then
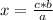
The rule of three will then be applied to calculate the limiting reagent as follows: if 124 g of phosphorus react with 96 g of oxygen by stoichiometry, 75.3 g (data) of phosphorus will react with how many grams of oxygen?
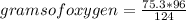
grams of oxygen= 58.3
But as data, you have only 38.7 grams of oxygen available. This indicates that the oxygen will be the limiting reagent.
Then the calculations will be made from the available 38.7 grams of the limiting reagent.
A rule of three applies: if 96 g of O₂ produce 220 grams of P₄O₆ per stoichiometry, when 38.7 grams of O₂ react how many grams of P₄O₆ will they form?
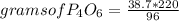
grams of P₄O₆= 88.68
So, knowing that 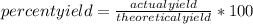 by definition.
by definition.
The theoretical yield of a reaction is the amount of product that would be formed if the reagents were completely consumed. The actual yield is what you really get from the product, because many times the reagents do not react completely, as in this case, where the P₄O₆ does not react completely. The actual yield of a reaction will always be equal to or less than the theoretical yield.
Then:
actual yield: 43.3 gtheorical yield: 88.68 g
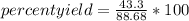
percent yield= 48.82%
Finally, the percent yield for the reaction is 48.82%





























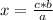
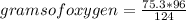
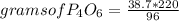
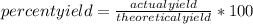 by definition.
by definition.