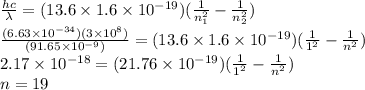Physics, 26.12.2019 23:31 algahimnada
Light shines through atomic hydrogen gas. it is seen that the gas absorbs light readily at a wavelength of 91.65 nm. what is the level to which the hydrogen is being excited by the absorption of light of this wavelength? assume that most of the atoms in the gas are in the lowest level.

Answers: 1


Another question on Physics

Physics, 22.06.2019 06:20
Atwo-stage air compressor operates at steady state, compression 10m^3/min of air from 100 kpa and 300k to 1200 kpa. an intercooler between the two stages cools the air to 300k at a constant pressure of 350 kpa. the compression processes are isentropic. a) calculate the power required to run the compressor, in kw b) compare the result to the power required for isentropic compression from the same inlet state to the same final pressure.
Answers: 1

Physics, 22.06.2019 06:30
2kg of refrigerant 134a undergoes a polytropic process in a piston-cylinder assembly from an initial state of saturated vapor at 2 bar to a final state of 12 bar, 80 degree c. a)determine the work for the process in kj. b)sketch the process on a p-v diagram.
Answers: 2

Physics, 22.06.2019 09:30
(**i would really appreciate if this was answered soon** ) which pair of quantities includes one quantity that increases as the other decreases during simple harmonic motion?
Answers: 3

Physics, 22.06.2019 13:50
Observations show that interstellar clouds can have almost any shape and
Answers: 1
You know the right answer?
Light shines through atomic hydrogen gas. it is seen that the gas absorbs light readily at a wavelen...
Questions

Spanish, 25.03.2020 06:38



Mathematics, 25.03.2020 06:38







Biology, 25.03.2020 06:38




Chemistry, 25.03.2020 06:38




Mathematics, 25.03.2020 06:38

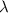 = Wavelength of the light absorbed = 91.65 nm = 91.65 x 10⁻⁹ m
= Wavelength of the light absorbed = 91.65 nm = 91.65 x 10⁻⁹ m 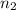 = nth level = n
= nth level = n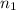 = 1
= 1