
Suppose you have 600.0 grams of room temperature water (20.0 degrees celsius) in a thermos. you drop 90.0 grams of ice at 0.00 degrees celsius into the thermos and shut the lid. (a) what is the equilibrium temperature of the system? (b) how much ice is left (in grams)?

Answers: 1


Another question on Physics

Physics, 21.06.2019 15:00
Dry ice is solid carbon dioxide that is used in the transport of food and medicine. dry ice becomes a gas at –78.5°c without becoming a liquid first.
Answers: 3

Physics, 21.06.2019 21:40
This problem has been solved! see the answera non-uniform fire escape ladder is 6.0m long whenextended to the icy alley below. it is held at the top by africtionless pivot, and there is neglibible frictional force fromthe icy surface at the bottom. the ladder weighs 250n, and it'scenter of gravity is 2.0m along the ladder from the bottom. amother and child of total weight 750n are on the ladder 1.5m fromthe pivot. the ladder makes an angle θ with the horizontal.find the magnitude and direction ofa) the force exerted by the icy alley on the ladderb) the force exerted by the ladder on the pivotc) do your answers in part a and b depend on the angle?
Answers: 3

Physics, 22.06.2019 01:00
An incident ray through the focal point of a spherical mirror will: reflect through the center of curvature. reflect back through the focal point. reflect parallel to the principle axis. reflect the same direction as the incident ray.
Answers: 1

Physics, 22.06.2019 07:00
Critical mass is the of material required to produce a chain reaction. a.) minimum amount b.) atomic mass c.) precise amount d.) maximum amount
Answers: 1
You know the right answer?
Suppose you have 600.0 grams of room temperature water (20.0 degrees celsius) in a thermos. you drop...
Questions


Mathematics, 02.04.2020 01:14

Mathematics, 02.04.2020 01:14

World Languages, 02.04.2020 01:14



History, 02.04.2020 01:14



Mathematics, 02.04.2020 01:14

Mathematics, 02.04.2020 01:14







Geography, 02.04.2020 01:15


Mathematics, 02.04.2020 01:15

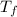 = 7.02 ° C
= 7.02 ° C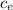 (T₀ -
(T₀ -

