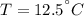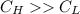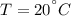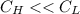Answers: 3


Another question on Physics

Physics, 22.06.2019 03:30
As part of an industrial process, air as an ideal gas at 10 bar, 400k expands at steady state through a valve to a pressure of 4 bar. the mass flow rate of air is 0.5 kg/s. the air then passes through a heat exchanger where it is cooled to a temperature of 295k with negligible change in pressure. the valve can be modeled as a throttling process, and kinetic and potential energy effects can be neglected. (a) for a control volume enclosing the valve and heat exchanger and enough of the local surroundings that the heat transfer occurs at the ambient temperature of 295 k, determine the rate of entropy production, in kw/k. (b) if the expansion valve were replaced by an adiabatic turbine operating isentropically, what would be the entropy production? compare the results of parts (a) and (b) and discuss.
Answers: 3

Physics, 22.06.2019 03:30
Calculate the mass of an object that has a momentum of 100kg x m/sec and velocity of 4 m/sec
Answers: 1


You know the right answer?
Energy is transferred as the heat between two objects, one with a temperature of 5 C and the other w...
Questions


Mathematics, 12.09.2019 05:10


History, 12.09.2019 05:10

Mathematics, 12.09.2019 05:10

Chemistry, 12.09.2019 05:10

Social Studies, 12.09.2019 05:10

Mathematics, 12.09.2019 05:10


English, 12.09.2019 05:10









Computers and Technology, 12.09.2019 05:10

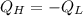
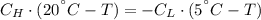
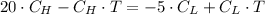
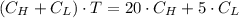
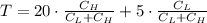
![C_{H} = C_{L]](/tpl/images/0541/2408/42956.png)