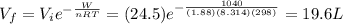Physics, 17.03.2020 03:29 landowatson123
A 1.88-mole sample of an ideal gas is contracted at a constant temperature of 298 K. The initial volume is 24.5 L and the amount of work performed on it is 1040 J. What is the final volume? Let the ideal-gas constant R = 8.314 J/(mol • K).
20.2 L
19.6 L
22.5 L
30.6 L

Answers: 1


Another question on Physics

Physics, 22.06.2019 05:20
Suppose an objects initial velocity is 10m/s and it’s final velocity is 4 m/s. mass is constant. what can best be concluded about the object based in the work-energy theorem
Answers: 2

Physics, 22.06.2019 12:50
Which changes would result in a decrease in the gravitational force btween two objects? check all that apply
Answers: 1

Physics, 22.06.2019 15:50
The space between two 15-in.-long concentric cylinders is filled with glycerin (viscosity = 8.5 × 10-3 lb·s/ft2). the inner cylinder has a radius of 1 in. and the gap width between cylinders is 0.1 in. determine (a) the torque and (b) the power required to rotate the inner cylinder at 180 rev/min. the outer cylinder is fixed. assume the velocity distribution in the gap to be linear.
Answers: 2

Physics, 22.06.2019 16:00
What is friction? how does it affect the motion of an object?
Answers: 1
You know the right answer?
A 1.88-mole sample of an ideal gas is contracted at a constant temperature of 298 K. The initial vol...
Questions

Mathematics, 30.06.2019 18:10






Mathematics, 30.06.2019 18:10

Mathematics, 30.06.2019 18:10







History, 30.06.2019 18:10


Mathematics, 30.06.2019 18:10

History, 30.06.2019 18:10


Social Studies, 30.06.2019 18:10

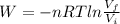
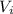 is the initial volume
is the initial volume is the final volume
is the final volume is the initial volume
is the initial volume