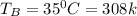Physics, 30.03.2020 17:57 twistedhyperboles
A 1-m3 tank containing air at 25 °C and 500 kPa is connected through a valve to another tank containing 5 kg of air at 35 °C and 200 kPa. Now the valve is opened, and the entire system is allowed to reach thermal equilibrium with the surroundings, which are at 20 °C. Determine the volume of the second tank and the final equilibrium pressure of air.

Answers: 2


Another question on Physics

Physics, 21.06.2019 12:50
If you mix chlorine lithium and nickel together which two elements would be the most reactive
Answers: 3

Physics, 22.06.2019 06:00
Imagine that someone pushes one marble toward a motionless marble. would there still be action-reaction forces involved in the collision? how might the marbles’ motions be changed? ?
Answers: 1

Physics, 22.06.2019 07:30
The charge on a charged sphere is: a)concentrated at its centerb)distributed uniformly throughout its volumec)clustered on its centerd)distributed uniformly over its surface
Answers: 1

You know the right answer?
A 1-m3 tank containing air at 25 °C and 500 kPa is connected through a valve to another tank contain...
Questions

Mathematics, 09.09.2021 19:00

Biology, 09.09.2021 19:00

English, 09.09.2021 19:00


Business, 09.09.2021 19:00


Social Studies, 09.09.2021 19:00


Engineering, 09.09.2021 19:00


Geography, 09.09.2021 19:00


Mathematics, 09.09.2021 19:00





Social Studies, 09.09.2021 19:00

Mathematics, 09.09.2021 19:00

Arts, 09.09.2021 19:00