
Physics, 18.08.2020 20:01 Teddybearnerd
An ideal gas, initially at a pressure of 11.2 atm and a temperature of 299 K, is allowed to expand adiabatically until its volume doubles.
Required:
What is the gas’s final pressure, in atmospheres, if the gas is diatomic?

Answers: 1


Another question on Physics



Physics, 22.06.2019 16:30
An astronaut in space cannot use a scale or balance to weigh objects because there is no gravity. but she does have devices to measure distance and time accurately. she knows her own mass is 77.4 kg , but she is unsure of the mass of a large gas canister in the airless rocket. when this canister is approaching her at 3.50 m/s , she pushes against it, which slows it down to 1.30 m/s (but does not reverse it) and gives her a speed of 2.60 m/s . what is the mass of the canister?
Answers: 1

Physics, 22.06.2019 17:00
Abowling ball rolling down the lane toward the pins has gravitational potential energy. a. no b. a lot of c. a little
Answers: 2
You know the right answer?
An ideal gas, initially at a pressure of 11.2 atm and a temperature of 299 K, is allowed to expand a...
Questions

Mathematics, 04.06.2021 14:00

Mathematics, 04.06.2021 14:00

Mathematics, 04.06.2021 14:00



Social Studies, 04.06.2021 14:00

Advanced Placement (AP), 04.06.2021 14:00


Chemistry, 04.06.2021 14:00



Health, 04.06.2021 14:00

Mathematics, 04.06.2021 14:00


Mathematics, 04.06.2021 14:00

Health, 04.06.2021 14:00


Mathematics, 04.06.2021 14:00


Biology, 04.06.2021 14:00

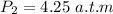

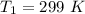
 Then the final volume will be
Then the final volume will be 
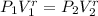
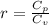
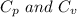 are the molar specific heat of a gas at constant pressure and the molar specific heat of a gas at constant volume with values
are the molar specific heat of a gas at constant pressure and the molar specific heat of a gas at constant volume with values 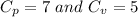
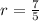
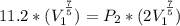
![P_2 = [\frac{1}{2} ]^{\frac{7}{5} } * 11.2](/tpl/images/0723/9998/ba19a.png)


