Physics, 18.02.2021 20:50 maddynichole2017
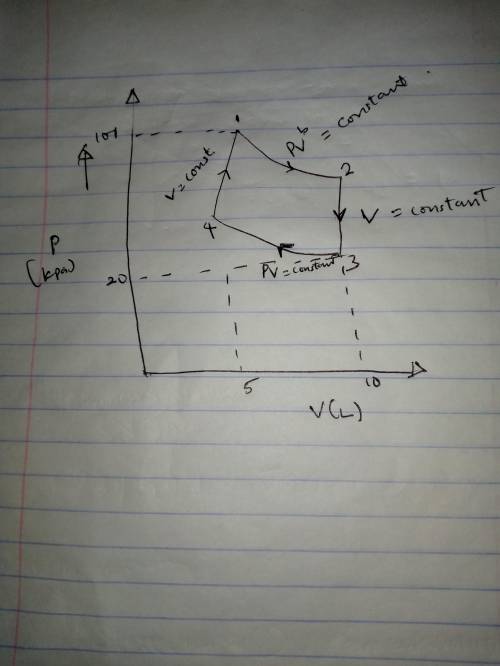
A mole of a monatomic ideal gas at point 1 (101 kPa, 5 L) is expanded adiabatically until the volume is doubled at point 2. Then it is cooled isochorically until the pressure is 20 kPa at point 3. The gas is now compressed isothermally until its volume is back to 5 L (point 4). Finally, the gas is heated isochorically to return to point 1.
a. Draw the four processes and label the points in the pV plane.
b. Calculate the work done going from 1 to 2.
c. Calculate the pressure and temperature at point 2.
d. Calculate the temperature at point 3.
e. Calculate the temperature and pressure and point 4.
f. Calculate the work done going from from 3 to 4.
g. Calculate the heat flow into the gas going from 3 to 4. g

Answers: 2


Another question on Physics

Physics, 22.06.2019 06:30
The mini-refrigerator fire was most likely caused by what type of wiring?
Answers: 2

Physics, 22.06.2019 14:00
Una carga puntual de 3 x 10-6 c se coloca a 12 cm de una segunda carga puntual de - 1,5 x 10-6 c. calcular la magnitud fuerza eléctrica entre las cargas
Answers: 2


Physics, 22.06.2019 17:40
You throw a baseball directly upward at time =0 at an initial speed of 14.9 m/s. what is the maximum height the ball reaches above where it leaves your hand? ignore air resistance and take =9.80 m/s2.
Answers: 1
You know the right answer?
A mole of a monatomic ideal gas at point 1 (101 kPa, 5 L) is expanded adiabatically until the volume...
Questions



Computers and Technology, 17.07.2019 13:00

English, 17.07.2019 13:00


History, 17.07.2019 13:00

History, 17.07.2019 13:00



Mathematics, 17.07.2019 13:00



History, 17.07.2019 13:00


Biology, 17.07.2019 13:00


Mathematics, 17.07.2019 13:00