
Physics, 26.03.2021 17:20 ArandomMexican3222
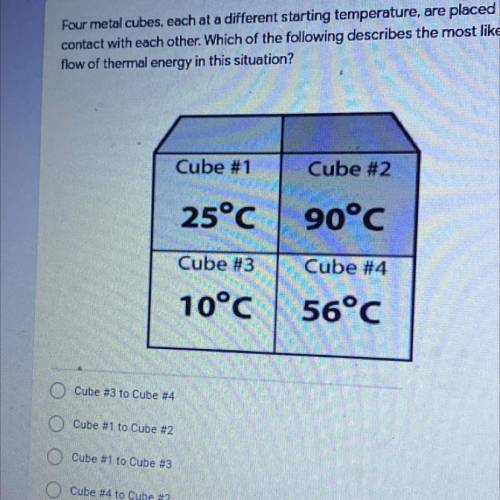
Four metal cubes, each at a different starting temperature, are placed in
contact with each other. Which of the following describes the most likely
flow of thermal energy in this situation?

Answers: 1


Another question on Physics

Physics, 22.06.2019 05:50
Acylinder with a movable piston contains 11.7 moles of a monatomic ideal gas at a pressure of 1.32×10^5 pa. the gas is initially at a temperature of 300 k. an electric heater adds 43200 j of energy into the gas while the piston moves in such a way that the pressure remains constant. cp=20.79 j k^−1 mol^−1 for a monatomic ideal gas, and that the number of gas molecules is equal to avogadro's number (6.022×10^23) times the number of moles of the gas. (a) what is the temperature of the gas after the energy is added? (b) what is the change in volume of the gas? (c) how much work is done by the gas during this process?
Answers: 3

Physics, 22.06.2019 14:30
70 give a real life example showing how sensory neurons work with the motor neurons
Answers: 2

Physics, 22.06.2019 18:50
8.29 two streams containing pyridine and acetic acid at 25°c are mixed and fed into a heat exchanger. due to the heat-of-mixing effect, it is desired to reduce the temperature after mixing to 25°c using a stream of chilled ethylene glycol as indicated in the diagram. calculate the mass flow rate of ethylene glycol needed. the heat capacity of ethylene glycol at these conditions is approximately 2.8 kj/(kg k), and the enthalpy change of mixing (δmixh) is given below.
Answers: 3

Physics, 22.06.2019 21:00
The velocity of a car traveling in a straight line increases from 0 meters/second to 30meters/second in 8 seconds. what is the average acceleration of the car?
Answers: 1
You know the right answer?
Four metal cubes, each at a different starting temperature, are placed in
contact with each other....
Questions






Mathematics, 09.03.2020 21:52



English, 09.03.2020 21:53





Mathematics, 09.03.2020 21:53

Biology, 09.03.2020 21:53

Biology, 09.03.2020 21:53







