
Physics, 17.09.2019 02:30 isaacgarcia0830
Calculate the freezing point of a solution of 40.0 g methyl salicylate, c7h6o2, dissolved in 800. g of benzene, c6h6. and the freezing point is 5.50°c for benzene. calculate the freezing point of a solution of 40.0 g methyl salicylate, c7h6o2, dissolved in 800. g of benzene, c6h6. and the freezing point is 5.50°c for benzene. 3.41°c -2.09°c 7.59°c 2.09°c

Answers: 1


Another question on Physics

Physics, 22.06.2019 00:30
What are the theoretical properties of a gas at a temperature of 0 kelvin?
Answers: 3

Physics, 22.06.2019 10:00
In a heat engine if 1000 j of heat enters the system the piston does 500 j of work, what is the final internal energy of the system if the initial energy was 2000 j? 1. write the equation 2.list out your known variables 3.plug the numbers into the equations 4.solve 5.write your solution statement that includes initial energy and final
Answers: 3

Physics, 22.06.2019 11:20
Suppose a diode consists of a cylindrical cathode with a radius of 6.200×10^−2 cm , mounted coaxially within a cylindrical anode with a radius of 0.5580 cm . the potential difference between the anode and cathode is 260 v . an electron leaves the surface of the cathode with zero initial speed (v initial=0). find its speed vfinal when it strikes the anode.
Answers: 1

Physics, 22.06.2019 14:30
Which of the following bonds would be most polar? a. c-i b. c-br c. c-cl d. c-f e. c-o
Answers: 1
You know the right answer?
Calculate the freezing point of a solution of 40.0 g methyl salicylate, c7h6o2, dissolved in 800. g...
Questions




Geography, 22.08.2019 18:10

History, 22.08.2019 18:10


Biology, 22.08.2019 18:10

Biology, 22.08.2019 18:10




Biology, 22.08.2019 18:10





Mathematics, 22.08.2019 18:10

Computers and Technology, 22.08.2019 18:10

Computers and Technology, 22.08.2019 18:10

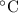 .
.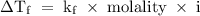
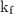 is the constant = 4.90 C/m (benzene), i = von't hoff factor = 1
is the constant = 4.90 C/m (benzene), i = von't hoff factor = 1 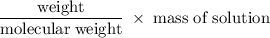
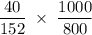
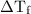 = 4.90
= 4.90  0.325
0.325 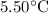 and the change in temperature is 1.5925
and the change in temperature is 1.5925 

