
Physics, 01.07.2021 15:30 kordejah348
A 3.10 mol sample of an ideal diatomic gas expands adiabatically from a volume of 0.1550 m3 to 0.742 m3 . Initially the pressure was 1.00 atm.(a) Determine the initial and final temperatures. initial Kfinal K(b) Determine the change in internal energy. J(c) Determine the heat lost by the gas. J(d) Determine the work done on the gas. J

Answers: 1


Another question on Physics

Physics, 22.06.2019 16:00
The electric potential v is constant everywhere within a certain region of space. which statement below is true? the choices are: the electric field is also constant (but not zero) within the region. a charged particle placed within the region will experience an electric force. the electric field is zero everywhere within the region. the electric field varies from place to place within the region.
Answers: 2

Physics, 22.06.2019 17:30
Heterogeneous mixture with larger particles that never settle is
Answers: 2


Physics, 22.06.2019 19:30
Which describes increasing the efficiency of energy resource
Answers: 2
You know the right answer?
A 3.10 mol sample of an ideal diatomic gas expands adiabatically from a volume of 0.1550 m3 to 0.742...
Questions




Mathematics, 06.03.2021 06:40






Chemistry, 06.03.2021 06:40

Spanish, 06.03.2021 06:40


Mathematics, 06.03.2021 06:40


Mathematics, 06.03.2021 06:40

Arts, 06.03.2021 06:40




Biology, 06.03.2021 06:40

 = 1 atm = 101325 pascal
= 1 atm = 101325 pascal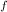 = ?
= ?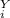 = P
= P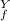
 V
V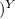
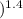
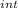 = nC
= nC ΔT
ΔT

