
Physics, 12.07.2021 21:20 naseersaad
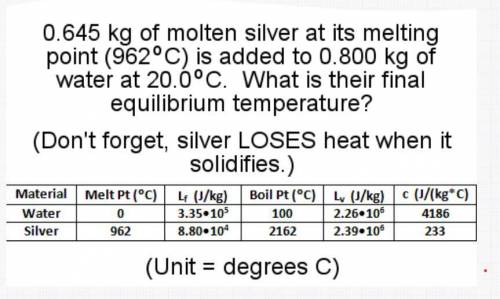
0.645 kg of molten silver at its melting point (962C) is added to 0.800 kg of water at 20.0 C. What is their final equilibrium temperature?
(Don't forget, silver LOSES heat when it solidifies.)
*ANY JOKE ANSWER WILL BE REPORTED AND YOU WILL BE BANNED*

Answers: 1


Another question on Physics

Physics, 22.06.2019 06:40
Light traveling in a medium with a refractive index 1.19 is incident on a plate of another medium with index of refraction 1.79. at what angle of incidence is the reflected light fully polarized?
Answers: 2

Physics, 22.06.2019 14:30
Explain what it means to view something from a frame of reference. provide an example that illustrates your explanation. (4 points)
Answers: 1


Physics, 22.06.2019 21:00
Which of the following statements comparing electron microscopy and light microscopy is false? which of the following statements comparing electron microscopy and light microscopy is false? both the electron microscope and the light microscope use the same wavelengths for illumination. images produced by light microscopes can be in color, whereas electron microscope images are black and white unless they are artificially colored. the electron microscope has greater resolution than the light microscope. electron microscopes can allow examination of viruses and internal cell structures, whereas light microscopes are limited to objects that are 0.5 micrometers and larger. request answer
Answers: 2
You know the right answer?
0.645 kg of molten silver at its melting point (962C) is added to 0.800 kg of water at 20.0 C. What...
Questions

Chemistry, 20.09.2020 09:01




Mathematics, 20.09.2020 09:01







Social Studies, 20.09.2020 09:01



Biology, 20.09.2020 09:01


Mathematics, 20.09.2020 09:01

Mathematics, 20.09.2020 09:01

Mathematics, 20.09.2020 09:01

Advanced Placement (AP), 20.09.2020 09:01



