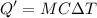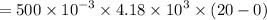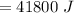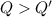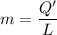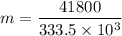Answers: 2


Another question on Physics

Physics, 21.06.2019 17:20
Research the neutrino using the internet and report your findings. in a nuclear decay, the nucleus of an atom splits apart. larger particles can be detected and their masses and velocities can be recorded. explain how the existence and properties of the neutrino could be predicted using the conservation laws.
Answers: 3

Physics, 22.06.2019 13:10
A0.750 kg aluminum pan is removed from the stove and plunged into a sink filled with 10.0 kg of water at 293 k. the water temperature quickly rises to 297 k. what was the initial temperature of the aluminum pan? the specific heat of aluminum is ca = 900 j/(kgk) and water is cw = 4190 j/(kgk)
Answers: 3

Physics, 22.06.2019 16:00
The field between two charged parallel plates is kept constant. if the two plates are brought closer together, the potential difference between the two plates either a) decrease b) does not change c) increase?
Answers: 3

Physics, 22.06.2019 18:00
Which statement is the best definition of an atom? a) anything that has mass and occupies space. b) the smallest particle that has the properties of an element. c) a substance that cannot be broken down chemically into simpler substances. eliminate d) the smallest unit of a substance that exhibits all of the properties characteristic of that substance.
Answers: 2
You know the right answer?
A 200 g piece of ice at 0°C is placed in 500 g of water at 20°C. The system is in a container of neg...
Questions

Mathematics, 21.12.2020 14:00

SAT, 21.12.2020 14:00


English, 21.12.2020 14:00

English, 21.12.2020 14:00



Business, 21.12.2020 14:00

Mathematics, 21.12.2020 14:00




Health, 21.12.2020 14:00

World Languages, 21.12.2020 14:00

English, 21.12.2020 14:00

Physics, 21.12.2020 14:00

English, 21.12.2020 14:00


English, 21.12.2020 14:00

Biology, 21.12.2020 14:00

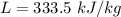
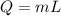
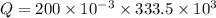
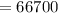 J
J