
Which statement best explains why the specific heat of water is higher than the specific heat of most other substances? a) due to its polarity and hydrogen bonding water can absorb heat without a significant temperature change. b) due to the ionic bonding in liquid water, it takes more heat energy to reach boiling. c) because water is a covalent compound, it takes a lot of energy to change its kinetic energy. d) water has a higher density value than most substances and requires more energy to change the temperature.

Answers: 1


Another question on Physics

Physics, 21.06.2019 18:40
An observer that stands still as the electron moves by him, will observe
Answers: 3

Physics, 21.06.2019 20:00
Spend time observing or thinking about events that involve matter and energy. which events can you explain? which events can’t you explain?
Answers: 1

Physics, 22.06.2019 03:30
The solar panels used by mark function because of the photoelectric effect. light shines on the cells causing electrons to be ejected from the metal, which produces an electric current. at night on mars, no light will fall on the solar cells and no electric current will be generated. according to your notes, what type of light is typically needed to cause the photoelectric effect? a)visible b)ultraviolet c)infrared
Answers: 1

Physics, 22.06.2019 07:00
Oxygen and hydrogen gas are at the same temperature t.what is the ratio of kinetic energies of oxygen molecule and hydrogen molecule if oxygen is 16 times heavier than hydrogen.
Answers: 3
You know the right answer?
Which statement best explains why the specific heat of water is higher than the specific heat of mos...
Questions





Mathematics, 06.08.2019 09:10

Mathematics, 06.08.2019 09:10





English, 06.08.2019 09:10

Mathematics, 06.08.2019 09:10



Mathematics, 06.08.2019 09:10





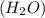 is formed of two hydrogen atoms and one oxygen atom. The atoms are have strong hydrogen bond. Also, water is a polar molecule. there is uneven distribution of density of electrons. This makes the bond even more stronger between the hydrogen atoms and oxygen. Hence, the heat required to raise the temperature of water is high compared to specific heat of other substances.
is formed of two hydrogen atoms and one oxygen atom. The atoms are have strong hydrogen bond. Also, water is a polar molecule. there is uneven distribution of density of electrons. This makes the bond even more stronger between the hydrogen atoms and oxygen. Hence, the heat required to raise the temperature of water is high compared to specific heat of other substances. 

