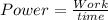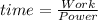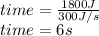Physics, 28.07.2019 22:30 micahmereus100
How much time does it take to use 300 w of power to do 1800 j of work? round your answer to the nearest whole number. the time is s.

Answers: 1


Another question on Physics

Physics, 22.06.2019 10:10
Apair of 10μf capacitors in a high-power laser are charged to 1.7 kv.a. what charge is stored in each capacitor? b. how much energy is stored in each capacitor?
Answers: 2

Physics, 22.06.2019 18:30
Ablock of mass m slides on a horizontal frictionless table with an initial speed v0 . it then compresses a spring of force constant k and is brought to rest. the acceleration of gravity is 9.8 m/s2. how much is the spring compressed x from its natural length? 1) x = v0*sqrt(k/(mg)) 2) x=v0*sqrt(m/k) 3) x=v0*((mk)/g) 4) x=v0*sqrt(k/m) 5) x=v0*(m/kg) 6) x=v0*sqrt((mg)/k) 7) x=(v0)^2/(2g) 8) x=v0*(k/(mg)) 9) x=(v0)^2/(2m) 10) x=v0*((mg)/k)
Answers: 3

Physics, 22.06.2019 20:20
An electron is trapped at a defect in a crystal. the defect may be modeled as a one-dimensional, rigid-walled box of width 1.00 nm. (a) sketch the wavefunctions and probability densities for the n 1 and n 2 states. (b) for the n 1 state, nd the probability of nding the electron between x1 0.15 nm and x2 0.35 nm, where x 0 is the left side of the box. (c) repeat (b) for the n 2 state. (d) calculate the energies in electron volts of the n 1 and n 2 states
Answers: 1

Physics, 22.06.2019 23:00
1700 j of energy is lost from 0.14 kg object , the temperature decreases from 50°c to 45°c what is the specific heat of this object, amd what is the material ?
Answers: 1
You know the right answer?
How much time does it take to use 300 w of power to do 1800 j of work? round your answer to the nea...
Questions

Mathematics, 14.08.2020 17:01


Social Studies, 14.08.2020 17:01



Mathematics, 14.08.2020 17:01



Mathematics, 14.08.2020 17:01

Mathematics, 14.08.2020 17:01

Mathematics, 14.08.2020 17:01


Mathematics, 14.08.2020 17:01



Chemistry, 14.08.2020 17:01


English, 14.08.2020 17:01

Mathematics, 14.08.2020 17:01

Biology, 14.08.2020 17:01