
Chemistry, 12.07.2019 09:30 qudoniselmore0
Complete combustion of 1.5 g of fructose a sugar that contains carbon, hydrogen, and oxygen yields 2.2 g of carbon dioxide and .9 g of water. determine the empirical formula of fructose

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:00
Ineed to find the answer of this question because i dont understand it
Answers: 1

Chemistry, 22.06.2019 19:00
Nan element’s square on the periodic table, the number with the greatest numerical value represents the
Answers: 3

Chemistry, 23.06.2019 00:20
Which diagram represents the phase tha occurs after a solid melts?
Answers: 1

Chemistry, 23.06.2019 05:50
What are the coefficients to balance the following equation? ba+br=babr2
Answers: 1
You know the right answer?
Complete combustion of 1.5 g of fructose a sugar that contains carbon, hydrogen, and oxygen yields 2...
Questions





Computers and Technology, 17.04.2020 01:10


History, 17.04.2020 01:10







Mathematics, 17.04.2020 01:11


Mathematics, 17.04.2020 01:11


Arts, 17.04.2020 01:11

Arts, 17.04.2020 01:11

Mathematics, 17.04.2020 01:11

 .
.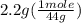 = 0.05 mole
= 0.05 mole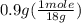 = 0.05 mole
= 0.05 mole has one C means the mole ratio of
has one C means the mole ratio of  has two H means the mole ratio of
has two H means the mole ratio of 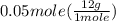 = 0.6 g
= 0.6 g = 0.1g
= 0.1g = 0.056 moles
= 0.056 moles = 1
= 1 = 2
= 2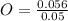 = 1
= 1

