

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:00
Given the following reaction: c3h8+5o2=3co2+4h20 how many grams of co2 will be produced 7 g of c3h8 and 98 g of o2
Answers: 1

Chemistry, 22.06.2019 23:00
What does a numerical subscript following an element in a chemical formula mean?
Answers: 1

Chemistry, 23.06.2019 00:50
What is the enthalpy of combustion (per mole) of c4h10 (g)? –2,657.5 kj/mol –5315.0 kj/mol –509.7 kj/mol –254.8 kj/mol
Answers: 1

Chemistry, 23.06.2019 01:30
Which of the following statements is true about energy quantization at the atomic level? electrons in the outermost orbits are the most stable. electrons in all the orbits around the nucleus have the same amount of energy. electrons in the orbit closest to the nucleus have the least amount of energy. electrons absorb or release the same amount of energy independent of the energy levels.
Answers: 1
You know the right answer?
Determine the enthalpy change of the following reaction: co + h2o -> h2 + co2 given enthalpies...
Questions

English, 12.08.2020 08:01







Computers and Technology, 12.08.2020 08:01












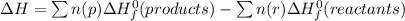
![\Delta H = [1\Delta H_{f}^{0}(H2)+1\Delta H_{f}^{0}(CO2)]-[1\Delta H_{f}^{0}(CO)+1\Delta H_{f}^{0}(H2O)]](/tpl/images/0034/1721/21403.png)


