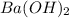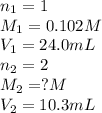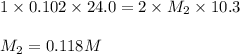Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Long term exposure to waves can cause sunburns and skin cancer. a) visible b) infrared c) gamma rays d) ultraviole
Answers: 1

Chemistry, 22.06.2019 17:20
The small bags of silica gel you often see in a new shoe box are placed there to control humidity. despite its name, silica gel is a solid. it is a chemically inert, highly porous, amorphous form of sio2. because water vapor readily adsorbs onto the surface of silica gel, it acts as a desiccant. despite not knowing mechanistic details of the adsorption of water onto silica gel, from the information provided you should be able to make an educated guess about the thermodynamic characteristics of the process. predict the signs for δg, δh, and δs for the adsorption of water.
Answers: 2


You know the right answer?
An aqueous solution of barium hydroxide is standardized by titration with a 0.102 m solution of perc...
Questions

English, 27.04.2020 02:28





History, 27.04.2020 02:28

History, 27.04.2020 02:28

Chemistry, 27.04.2020 02:28

Mathematics, 27.04.2020 02:28

Mathematics, 27.04.2020 02:28

Biology, 27.04.2020 02:28


Chemistry, 27.04.2020 02:28



English, 27.04.2020 02:28

Chemistry, 27.04.2020 02:28



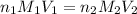
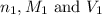 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 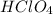
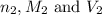 are the n-factor, molarity and volume of base which is
are the n-factor, molarity and volume of base which is