
Calculate the amount of heat energy in kj required to convert 45.0 g of ice at -15.5'c to steam at 124.0°c. (cwater 118 jig'c, gee 2.03 jig c, g team jig c, molar heat of fusion of ice 6.01 * 10 j/mol; molar heat of vaporization of liquid water 4.07 * 10*j/mol 202 short answer toolbar navigation b i v s e 1 e a a this question will be sent to your instructor for grading

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:30
If i make a solution by adding 83grams of sodium hydroxide to 750ml i’d water what is the molarity of sodium hydroxide
Answers: 1

Chemistry, 22.06.2019 11:00
Iron (3) oxide will decompose in the presence of hydrogen gas and heater to produced iron and digydrogen monoxide white a balanced chemical equation
Answers: 1

Chemistry, 22.06.2019 22:00
Does the number of ions in solution increase, decrease, or remain constant? it continuously decreases. it continuously increases. it decreases at first, then increases. it increases at first, then decreases.
Answers: 3

Chemistry, 22.06.2019 23:00
Which organism develops breathing organism develops breathing organs from pharyngeal arches? shark, spider, sea star, sea horse
Answers: 2
You know the right answer?
Calculate the amount of heat energy in kj required to convert 45.0 g of ice at -15.5'c to steam at 1...
Questions


Biology, 30.07.2019 10:00

Social Studies, 30.07.2019 10:00


Business, 30.07.2019 10:00

Business, 30.07.2019 10:00




Biology, 30.07.2019 10:00

Biology, 30.07.2019 10:00


Business, 30.07.2019 10:00



Biology, 30.07.2019 10:00

History, 30.07.2019 10:00

Biology, 30.07.2019 10:00


Social Studies, 30.07.2019 10:00

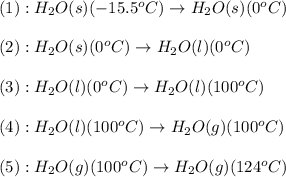
![\Delta H=[m\times c_{p,s}\times (T_{final}-T_{initial})]+n\times \Delta H_{fusion}+[m\times c_{p,l}\times (T_{final}-T_{initial})]+n\times \Delta H_{vap}+[m\times c_{p,g}\times (T_{final}-T_{initial})]](/tpl/images/0213/6323/e4ef0.png)
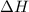 = enthalpy change or heat required = ?
= enthalpy change or heat required = ?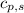 = specific heat of solid water =
= specific heat of solid water = 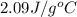
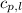 = specific heat of liquid water =
= specific heat of liquid water = 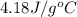
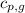 = specific heat of liquid water =
= specific heat of liquid water = 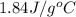
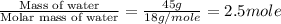
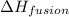 = enthalpy change for fusion = 6.01 KJ/mole = 6010 J/mole
= enthalpy change for fusion = 6.01 KJ/mole = 6010 J/mole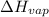 = enthalpy change for vaporization = 40.67 KJ/mole = 40670 J/mole
= enthalpy change for vaporization = 40.67 KJ/mole = 40670 J/mole![\Delta H=[45g\times 4.18J/gK\times (0-(-15.5))^oC]+2.5mole\times 6010J/mole+[45g\times 2.09J/gK\times (100-0)^oC]+2.5mole\times 40670J/mole+[45g\times 1.84J/gK\times (124-100)^oC]](/tpl/images/0213/6323/555ef.png)
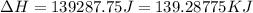 (1 KJ = 1000 J)
(1 KJ = 1000 J)

