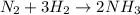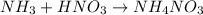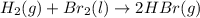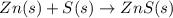Chemistry, 02.09.2019 21:30 Spencerg325
Write balanced chemical equation for the reactions used to prepare each of the following compounds from the given starting material(s). in some cases, additional reactants may be required.
(a) solid ammonium nitrate from gaseous molecule of nitrogen viaa two step process (first reduce the nitrogen to ammonia than neutrlize the ammonium in an appropriate acid)
(b)gaseous hydrogen bromide liquid molecular bromin via one step redox reaction
(c)gaseous h2s from solid zn and s via a two step process(first a redox reaction between the starting material then reaction of the product with a strong acid)

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 21:00
The rate constant for the reaction below is 6.2 x 10−5 mol l−1 s −1. if the initial concentration of a is 0.0500 m, what is its concentration after 115 s?
Answers: 1

Chemistry, 23.06.2019 07:00
Introduction of drugs into the gastrointestinal tract is a form of administration. a. enteral b. topical c. parenteral d. inhalation
Answers: 1

Chemistry, 23.06.2019 08:30
Kelly has come up with an explanation for why her sister is sometimes in a good mood and other times in a bad mood. she speculates that it is based on the hours of sleep her sister got the previous night. this explanation for her sister's behaviors is an example of a(n)
Answers: 3
You know the right answer?
Write balanced chemical equation for the reactions used to prepare each of the following compounds f...
Questions

Mathematics, 05.05.2020 00:35


English, 05.05.2020 00:35

Physics, 05.05.2020 00:35

History, 05.05.2020 00:35

Chemistry, 05.05.2020 00:35


Mathematics, 05.05.2020 00:35






English, 05.05.2020 00:35

Mathematics, 05.05.2020 00:35



English, 05.05.2020 00:35


English, 05.05.2020 00:35