

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 07:30
Using data from seismic waves, geologists have learned that earth’s interior is made up of several
Answers: 3


Chemistry, 23.06.2019 00:00
(04.05 hc) analyze the given diagram of the carbon cycle below. part 1: which compound does c represent? part 2: name a process that could release this compound into the air. part 3: explain how the elements that form it are conserved during the carbon cycle. use complete sentences to explain your answer. justify how this compound was created from a recycling of carbon in the carbon cycle. use complete sentences to explain your answer.
Answers: 3
You know the right answer?
When nh3(g) reacts with o2(g), the products of the combustion are no(g) and h2o(g). what volume of o...
Questions










English, 14.01.2021 19:40






Chemistry, 14.01.2021 19:40

Advanced Placement (AP), 14.01.2021 19:40

Chemistry, 14.01.2021 19:40


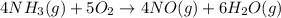
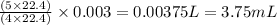 of oxygen gas
of oxygen gas


