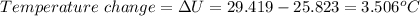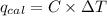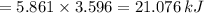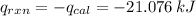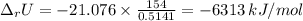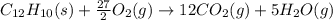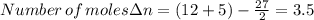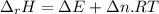Chemistry, 08.12.2019 20:31 gabrielaelisa224
When 0.5141 g of biphenyl (c12h10) undergoes combustion in a bomb calorimeter, the temperature rises from 25.823 °c to 29.419 °c. find δru and δrh for the combustion of biphenyl in kj mol−1 at 298 k. the heat capacity of the bomb calorimeter, determined in a separate experiment, is 5.861 kj °c−1.

Answers: 1


Another question on Chemistry



Chemistry, 22.06.2019 17:40
How much heat is added if 0.814g of water increase in temperature by 0.351 degree c?
Answers: 3

Chemistry, 22.06.2019 20:30
A40 kilogram skier starts at the top of a 12 meter high slope. at the bottom, she is travelling 10 meters per second. how much energy does she lose to friction
Answers: 2
You know the right answer?
When 0.5141 g of biphenyl (c12h10) undergoes combustion in a bomb calorimeter, the temperature rises...
Questions


Mathematics, 23.02.2021 08:30

Chemistry, 23.02.2021 08:30




History, 23.02.2021 08:30

Mathematics, 23.02.2021 08:30

Physics, 23.02.2021 08:30

Physics, 23.02.2021 08:30


History, 23.02.2021 08:30



Mathematics, 23.02.2021 08:30

Geography, 23.02.2021 08:30

Mathematics, 23.02.2021 08:30

Arts, 23.02.2021 08:30

Biology, 23.02.2021 08:30

Advanced Placement (AP), 23.02.2021 08:30

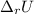 of the reaction is -6313 kJ/mol
of the reaction is -6313 kJ/mol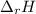 of the reaction is -6312 kJ/mol
of the reaction is -6312 kJ/mol