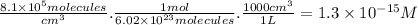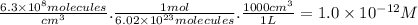The degradation of cf3ch2f (an hfc) by oh radicals in the troposphere is first order in each reactant and has a rate constant of k = 1.6 x 10^8 m^-1s^-1 at 4°c.
part a) if the tropospheric concentrations of oh and cf3ch2f are 8.1 x 10^5 and 6.3 x 10^8 molecules/cm^3, respectively, what is the rate of reaction at this temperature in m/s?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:00
One of the few xenon compounds that form is cesium xenon heptafluoride (csxef7). how many moles of csxef7 can be produced from the reaction of 13.0 mol cesium fluoride with 12.5 mol xenon hexafluoride? csf(s) + xef6(s) csxef7(s)
Answers: 1

Chemistry, 22.06.2019 10:30
Which of these is not an example of chemical weathering? a. iron-rich mineral rusting b. feldspar turning into clay c. limestone reacting with acid d. granite breaking up into sand
Answers: 1

Chemistry, 22.06.2019 14:50
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 9 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of tthe table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 9 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3

You know the right answer?
The degradation of cf3ch2f (an hfc) by oh radicals in the troposphere is first order in each reactan...
Questions

Computers and Technology, 26.11.2019 03:31










Biology, 26.11.2019 03:31






Computers and Technology, 26.11.2019 03:31