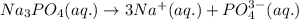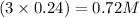Chemistry, 20.01.2020 18:31 ryleeyeagley03
The ionic compound, na3po4, will fully dissolve in water. if 19.68 g is dissolved in 504.0 ml water, find the following ion concentrations.
part 1: the concentration of the na+ is:
part 2: the concentration of the po43- is:

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 20:00
Suppose that some of the compound spilled out of the crucible after it was heated. would that cause the percent by mass of water in the compound determined by the experiment to be too low, too high, or unchanged? briefly explain your answer.
Answers: 1


Chemistry, 23.06.2019 03:40
The following questions a24 - a26 relate to 100 ml of 0.0150 m solution of benzoic acid (c6h3cooh). ka(c6h3cooh) = 6.4 x 10^-5. what is the ph of the solution after the addition of 1 x 10^-3 moles of naoh? you may assume no volume change to the solution upon addition of the naoh.
Answers: 2

Chemistry, 23.06.2019 06:00
Which factor is likely to impact the possible number of compounds? presence of unlimited number of elements in the periodic table the inability of atoms to align perfectly with other atoms the ability of all elements to react with every other element all elements being equally reactive
Answers: 2
You know the right answer?
The ionic compound, na3po4, will fully dissolve in water. if 19.68 g is dissolved in 504.0 ml water,...
Questions


History, 23.08.2019 17:10

Chemistry, 23.08.2019 17:10


English, 23.08.2019 17:10




Chemistry, 23.08.2019 17:10


Physics, 23.08.2019 17:10


History, 23.08.2019 17:10


Mathematics, 23.08.2019 17:10


Computers and Technology, 23.08.2019 17:10



 is 0.72 M and 0.24 M respectively.
is 0.72 M and 0.24 M respectively.
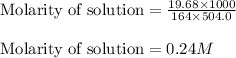
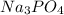 . Its ionization reaction follows:
. Its ionization reaction follows: