
Chemistry, 24.01.2020 01:31 savannahvargas512
An ionic compound mx3 is prepared according to the following unbalanced chemical equation. m + x2 gives mx3, a 0.105-g sample of x2 contains 8.92 x 10^20 molecules. the compound mx3 consists of 54.47% x by mass. what are the identities of m and x, and what is the correct name for mx3? starting with 1.00 g each of m and x2, what mass of mx3 can be prepared?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 15:00
Which element in the third period would you expect to have the larger atomic radius, sodium (na) or sulfur (s)? a. sodium, because it has a higher effective nuclear charge attracting electrons in fewer energy levels. b. sodium, because it has fewer protons attracting electrons in the same energy levels. c. sulfur, because it has more protons attracting electrons in more energy levels. d. sulfur, because it has a higher effective nuclear charge attracting electrons in the same energy levels.
Answers: 2

Chemistry, 22.06.2019 07:00
In the cathode ray tube experiment, j. j. thomson passed an electric current through different gases inside a cathode ray tube in the presence of an electric field. in which two ways did this experiment change scientists’ understanding of the atom?
Answers: 2

Chemistry, 23.06.2019 01:00
If a sample of radioactive isotopes takes 600 minutes to decay from 400 grams to 50 grams, what is the half-life of the isotope?
Answers: 1

Chemistry, 23.06.2019 03:00
Abaker touches a pie right after taking it out of the oven. which statement best explains why the pie feels hot?
Answers: 1
You know the right answer?
An ionic compound mx3 is prepared according to the following unbalanced chemical equation. m + x2 gi...
Questions


Mathematics, 26.07.2019 09:00



Mathematics, 26.07.2019 09:00


Biology, 26.07.2019 09:00



Geography, 26.07.2019 09:00

History, 26.07.2019 09:00






World Languages, 26.07.2019 09:00



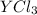 .
.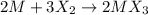
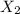 =n
=n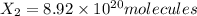
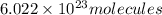
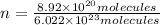
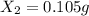
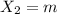
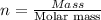
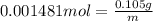
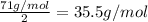
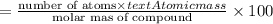
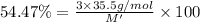
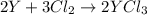
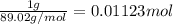
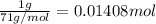
 of Y.
of Y.


