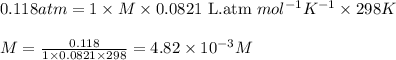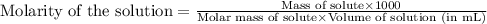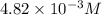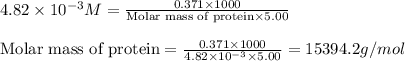Chemistry, 24.01.2020 01:31 niyyyareligion
371. mg of an unknown protein are dissolved in enough solvent to make 5.00 ml of solution. the osmotic pressure of this solution is measured to be at 0.118 atm at 25 c
calculate the molar mass of the protein.

Answers: 1


Another question on Chemistry

Chemistry, 23.06.2019 00:30
An ice cube with a volume of 45.0ml and a density of 0.9000g/cm3 floats in a liquid with a density of 1.36g/ml. what volume of the cube is submerged in the liquid
Answers: 3

Chemistry, 23.06.2019 01:00
Which of the following is a physical change? a.burning a piece of wood b.sawing a piece of wood in half c.rust forming on an iron fence d.a copper roof changing color from orange to green
Answers: 1

Chemistry, 23.06.2019 06:00
What physical property of gold makes panning a useful way to get gold from streams?
Answers: 2

Chemistry, 23.06.2019 07:00
Under what conditions will a gas be most likely to exhibit the ideal gas properties predicted by the ideal gas law? 1)high pressures and high temperature, because particles are forced closer together with higher kinetic energy, so intermolecular forces of attraction are weaker 2)high pressure and low temperature, because particles are forced closer together and moving slower, so the volume of the particles is less significant 3) low pressure and high temperature, because particles are spread farther apart and moving faster, so the intermolecular forces of attraction are weaker 4)low pressure and low temperature, because particles are spread farther apart with lower kinetic energy, so the volume of the particles is less significant
Answers: 2
You know the right answer?
371. mg of an unknown protein are dissolved in enough solvent to make 5.00 ml of solution. the osmot...
Questions








English, 10.03.2020 04:28












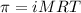
 = osmotic pressure of the solution = 0.118 atm
= osmotic pressure of the solution = 0.118 atm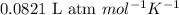
![25^oC=[273+25]=298K](/tpl/images/0468/1226/6a9f9.png)