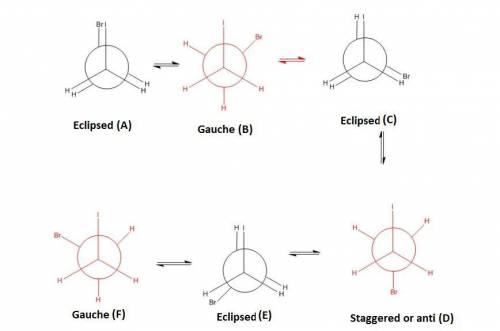
Draw 1-bromo-2-iodoethane. Looking through the C-C bond, draw all 6 rotational conformations as Newman projections. Label the conformations as anti, gauche or eclipsed. Then, rank them in terms of lowest to highest energy. Explain your reasoning in 2-3 sentences.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Which type of bond is present in hydrogen sulfide (h2s)? the table of electronegativities is given. a. hydrogen b. ionic c. nonpolar covalent d. polar covalent
Answers: 1

Chemistry, 22.06.2019 00:10
According to the diagram; a) identify the anode of the cell and write the half-reaction that occurs there b) write the overall equation for the reaction that occurs as the cell operates c) calculate the value of the standard cell potential ,e cell. d)write the shorthand notation of the cell above e)indicate the flow of the electrons on the diagram
Answers: 3

Chemistry, 22.06.2019 04:00
Seltzer water is created by placing water under pressure with carbon dioxide gas. which of the following statements best describe seltzer water: a. the solution will be slightly acidic b. the solution will be slightly basic. the solution will be strongly acidic. d. the solution will be strongly basic. e. the solution will be neutral
Answers: 3

Chemistry, 22.06.2019 05:30
What is the mass of each element in a 324.8 sample of co2
Answers: 1
You know the right answer?
Draw 1-bromo-2-iodoethane. Looking through the C-C bond, draw all 6 rotational conformations as Newm...
Questions












Computers and Technology, 24.02.2020 16:44



Mathematics, 24.02.2020 16:45


Chemistry, 24.02.2020 16:45