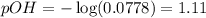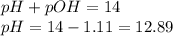Chemistry, 02.04.2020 03:21 brattymoo1009
A chemist dissolves 697. mg of pure potassium hydroxide in enough water to make up 160. mL of solution. Calculate the pH of the solution.
(The temperature of the solution is 25 °C.)
Be sure your answer has the correct number of significant digits.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
What must happen before a body cell can begin mitotic cell division
Answers: 2

Chemistry, 22.06.2019 08:40
Write the formula for the following chemicals. 7. e. trinitrogen tetraoxide a calcium phosphate f. magnesium acetate b. potassium sulfide g nickel(iii) cyanide c carbon dioxide h. silver sulfate d. cobalt(ii) chloride
Answers: 1

Chemistry, 22.06.2019 10:00
Ahydrogen atom has 1 electron. how many bonds can hydrogen form? a) 1 b) 2 c) 3 d) 4 e) 5
Answers: 3

Chemistry, 22.06.2019 19:00
Which change to the system wood cause the freely-moving piston to lower?
Answers: 1
You know the right answer?
A chemist dissolves 697. mg of pure potassium hydroxide in enough water to make up 160. mL of soluti...
Questions

English, 25.09.2019 00:10

Mathematics, 25.09.2019 00:10


Mathematics, 25.09.2019 00:10


SAT, 25.09.2019 00:10






English, 25.09.2019 00:10








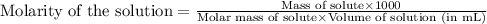
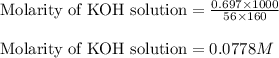
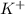 ions and 1 mole of
ions and 1 mole of 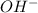 ions
ions![pOH=-\log[OH^-]](/tpl/images/0577/3716/fe336.png)
![[OH^-]=0.0778M](/tpl/images/0577/3716/a39b0.png)