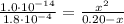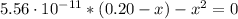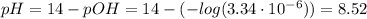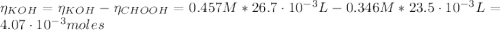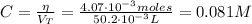Chemistry, 16.04.2020 00:36 RoxanneDuartee
Determine the pH during the titration of 23.5 mL of 0.346 M formic acid (Ka = 1.8×10-4) by 0.457 M KOH at the following points.
(a) Before the addition of any KOH
(b) After the addition of 4.00 mL of KOH
(c) At the half-equivalence point (the titration midpoint)
(d) At the equivalence point
(e) After the addition of 26.7 mL of KOH

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 10:00
What is the atomic mass of an atom that has 6 protons, 6 neutrons, and 6 electrons? a) 6 b) 8 c) + 1 d) 12 e) 18
Answers: 1

Chemistry, 22.06.2019 20:00
There are two steps in the usual industrial preparation of acrylic acid, the immediate precursor of several useful plastics. in the first step, calcium carbide and water react to form acetylene and calcium hydroxide: cac2 (s) + 2h2o (g) → c2h2 (g) + caoh2 (s) =δh−414.kj in the second step, acetylene, carbon dioxide and water react to form acrylic acid: 6c2h2 (g) + 3co2 (g) + 4h2o (g) → 5ch2chco2h (g) =δh132.kj calculate the net change in enthalpy for the formation of one mole of acrylic acid from calcium carbide, water and carbon dioxide from these reactions. round your answer to the nearest kj .
Answers: 3

Chemistry, 22.06.2019 20:30
Consider the following unbalanced equation for the combustion of hexane: αc6h14(g)+βo2(g)→γco2(g)+δh2o(g) part a balance the equation. give your answer as an ordered set of numbers α, β, γ, use the least possible integers for the coefficients. α α , β, γ, δ = nothing request answer part b determine how many moles of o2 are required to react completely with 5.6 moles c6h14. express your answer using two significant figures. n n = nothing mol request answer provide feedback
Answers: 2
You know the right answer?
Determine the pH during the titration of 23.5 mL of 0.346 M formic acid (Ka = 1.8×10-4) by 0.457 M K...
Questions




Social Studies, 07.07.2020 17:01




Computers and Technology, 07.07.2020 17:01






Mathematics, 07.07.2020 17:01





Computers and Technology, 07.07.2020 17:01

![pH = -log([H_{3}O^{+}])](/tpl/images/0603/8843/6ab72.png) (1)
(1) ![K_{a} = \frac{[CHOO^{-}][H_{3}O^{+}]}{[CHOOH]}](/tpl/images/0603/8843/c4333.png) (3)
(3)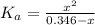
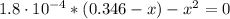 (4)
(4)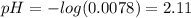
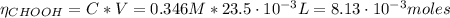
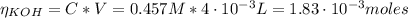
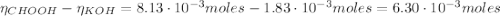
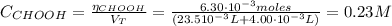
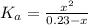
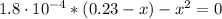
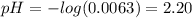
![pH = pKa + log(\frac{[CHOO^{-}]}{[CHOOH]})](/tpl/images/0603/8843/112dd.png)
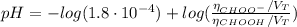
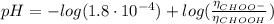
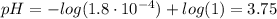
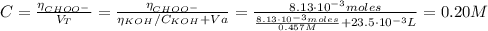
![K_{b} = \frac{[CHOOH][OH^{-}]}{[CHOO^{-}]}](/tpl/images/0603/8843/a45f9.png)
![\frac{K_{w}}{K_{a}} = \frac{[CHOOH][OH^{-}]}{[CHOO^{-}]}](/tpl/images/0603/8843/7611b.png)