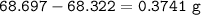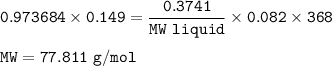Chemistry, 02.11.2020 14:10 joycewingate919
An empty 149 mL flask weighs 68.322 g before a sample of volatile liquid is added. The flask is then placed in a hot (95.0°C) water bath; the barometric pressure is 740 torr. The liquid vaporizes and the gas fills the flask. After cooling, flask and condensed liquid together weigh 68.697 g. What is the molar mass of the liquid?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Which of the following ocean acidification? are the most likely side effects of a ph less than 7.0 in the ocean b. more metal salts altering the ocean chemistry c. dissolution of the shells of marine organisms d. both a & b e. all of the above.
Answers: 3

Chemistry, 22.06.2019 18:30
When a device is used in a circuit in which the voltage is 81 v the current flowing through the device is 3 a what is the resistance of the device
Answers: 2

Chemistry, 22.06.2019 21:30
What is another way to determine mass times acceleration?
Answers: 1

Chemistry, 22.06.2019 22:00
8) warming your hands by a fire is an example if which heat transfer? a. conduction b. convection c. radiation d. none of these
Answers: 1
You know the right answer?
An empty 149 mL flask weighs 68.322 g before a sample of volatile liquid is added. The flask is then...
Questions

Mathematics, 29.05.2020 13:57



Mathematics, 29.05.2020 13:57

Mathematics, 29.05.2020 13:57

English, 29.05.2020 13:57




History, 29.05.2020 13:57

English, 29.05.2020 13:57

Mathematics, 29.05.2020 13:57


English, 29.05.2020 13:57



History, 29.05.2020 13:57