APPLY YOUR KNOWLEDGE
Graphite and diamond are different forms of the element carbon.
Graphite...

Chemistry, 22.01.2021 14:00 anacecilianr2325
APPLY YOUR KNOWLEDGE
Graphite and diamond are different forms of the element carbon.
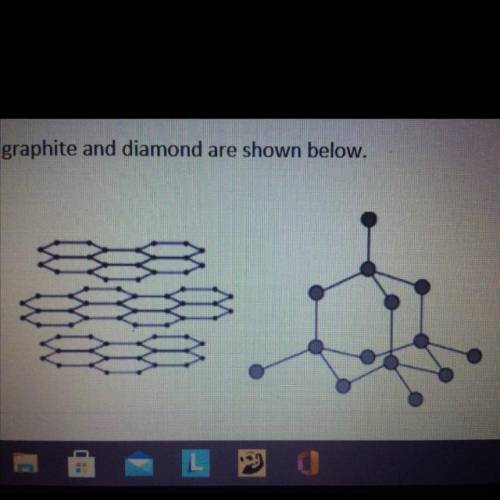
Graphite and diamond have different properties.
The structures of graphite and diamond are shown below.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Liv sheldon given the balanced equation for an organic reaction: c2h2 + 2cl2 → c2h2cl4 this reaction is best classified as *
Answers: 1

Chemistry, 22.06.2019 05:30
The climate of the continental united states is generally 1. tropical 2. temperate 3. arctic 4. highland
Answers: 1

Chemistry, 22.06.2019 10:50
Determine the empirical formula for succinic acid that is composed of 40.60% carbon, 5.18% hydrogen, and 54.22% oxygen.
Answers: 1

Chemistry, 22.06.2019 21:00
Which of these is an example of pseudoscience? a) predicting the time of sunrise based on data on position of earth b) predicting the date of the moon phases based on data on position of earth c) predicting eclipses based on the position of the sun and the moon d) predicting future events in a person's life based on the position of the moon
Answers: 1
You know the right answer?
Questions


Mathematics, 23.07.2019 04:00




Biology, 23.07.2019 04:00








Social Studies, 23.07.2019 04:00

History, 23.07.2019 04:00







