
Chemistry, 26.03.2021 22:50 barnhill4755
Which of these equations represent reactions that could be used in constructing an electrochemical cell? Check all that apply.
A. CH4 +2O2 → CO2 + 2H20
B. Cr + Cu^2+ ---> Cr^2+ + Cu
C. 2 Ag+ + Fe → 2Ag + Fe^2+
D. CI^- + Ag^+ → AgCI
E. NH3 +H^+ ---> NH4^4
just got it wrong, the answers are B and C. Just solved my own question
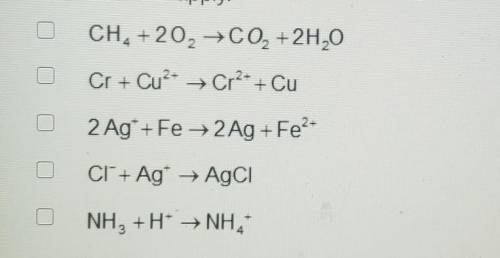

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 23:00
What is the most common reason for matter changing its state?
Answers: 1

Chemistry, 23.06.2019 00:30
You are attempting to recrystallize a crude product mixture. you add the appropriate amount of hot solvent and are allowing the solution to slowly cool to room temperature. however, at room temperature no crystals have appeared, which of the following methods should be used to induce crystallization? choose all correct answers. a) place the flask in an ice bath. b) swirl the contents of the flask. c) add a small seed crystal of the desired product. d) scratch the inside of the glassware using a stir rod. it can be multiple choices
Answers: 3

Chemistry, 23.06.2019 08:00
If the solubility of a gas in water is 1.22 g/l at 2.75 atm, what is its solubility (in g/l) at 1.0 atm?
Answers: 1

Chemistry, 23.06.2019 12:00
Explaining why atoms bondcomplete the sentence.atoms form chemical bonds to satisfy the rule and to become .
Answers: 1
You know the right answer?
Which of these equations represent reactions that could be used in constructing an electrochemical c...
Questions


Mathematics, 21.01.2021 14:00


Business, 21.01.2021 14:00

Mathematics, 21.01.2021 14:00


Mathematics, 21.01.2021 14:00

World Languages, 21.01.2021 14:00

Mathematics, 21.01.2021 14:00

Mathematics, 21.01.2021 14:00

Mathematics, 21.01.2021 14:00

Chemistry, 21.01.2021 14:00

Physics, 21.01.2021 14:00

Mathematics, 21.01.2021 14:00

Mathematics, 21.01.2021 14:00


Advanced Placement (AP), 21.01.2021 14:00

Social Studies, 21.01.2021 14:00




