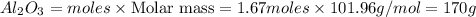Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Ibeg i need 20. a reaction produces 4.93 l of oxygen, but was supposed to produce 1 mol of oxygen. what is the percent yield?
Answers: 1

Chemistry, 22.06.2019 04:30
How much energy is made when a pice of wood burns. how do you know
Answers: 2

Chemistry, 22.06.2019 06:00
When a spring is compressed, the energy changes from kinetic to potential. which best describes what is causing this change?
Answers: 3

You know the right answer?
How many grams of Al2O3 can be formed when 3.33 moles of Al and 4.87 moles of O2 react according to...
Questions

Mathematics, 05.04.2021 14:00


Mathematics, 05.04.2021 14:00

Mathematics, 05.04.2021 14:00

Biology, 05.04.2021 14:00



Business, 05.04.2021 14:00

History, 05.04.2021 14:00

Mathematics, 05.04.2021 14:00

Spanish, 05.04.2021 14:00

Mathematics, 05.04.2021 14:00

Biology, 05.04.2021 14:00


Social Studies, 05.04.2021 14:00

Mathematics, 05.04.2021 14:00


Mathematics, 05.04.2021 14:00

Business, 05.04.2021 14:00

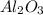
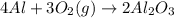
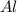 require = 3 moles of
require = 3 moles of 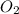
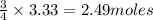 of
of 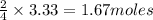 of
of