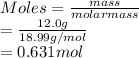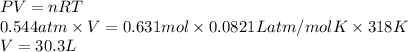Chemistry, 11.06.2021 06:50 bgallman153p71edg
If a sample of 12.0 grams of fluorine gas at 45.00C has a pressure of 0.544 atm, what is the volume of the container?
R = 0.0821 L atm/mol K
15.1 L
4.29 L
30.3 L
2.14 L

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 15:20
An alloy contains 66 g of pure zinc. what is the percentage of zinc in the alloy? express your answer to two significant figures and include the appropriate units.
Answers: 1

Chemistry, 22.06.2019 18:50
Which of the following is a conclusion that resulted from ernest rutherford’s scattering experiment? (will mark brainliest) a. the nucleus is negatively charged b. the atom is a dense solid and is indivisible c. the mass is conserved when atoms react chemically d. the nucleus is very small and the atom is mostly empty space
Answers: 3

Chemistry, 22.06.2019 21:30
What is the correct name for the compound cocl3? a) cobalt(i) chloride b) cobalt(i) chlorate c) cobalt(ii) chlorate d) cobalt(iii) chloride
Answers: 1

Chemistry, 22.06.2019 22:30
The vapor pressure of ethanol is 1.00 × 102 mmhg at 34.90°c. what is its vapor pressure at 61.61°c? (δhvap for ethanol is 39.3 kj/mol.)
Answers: 2
You know the right answer?
If a sample of 12.0 grams of fluorine gas at 45.00C has a pressure of 0.544 atm, what is the volume...
Questions




Physics, 20.09.2020 15:01


Mathematics, 20.09.2020 15:01



Physics, 20.09.2020 15:01







French, 20.09.2020 15:01


Mathematics, 20.09.2020 15:01


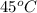 = (45 + 273) K = 318 K
= (45 + 273) K = 318 K