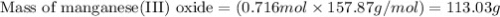Chemistry, 30.06.2021 07:20 brittanyelliott028
Using the balanced equation below,
how many grams of manganese(III)
oxide would be produced from the
complete reaction of 46.8 g of zinc?
Zn + 2MnO2 + H20 — Zn(OH)2 + Mn203


Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:30
Identify one disadvantage to each of the following models of electron configuration: -dot structures -arrow and line diagrams -written electron configurations type in your answer below. (answer) -dot structures do not show the distribution of electrons in orbitals and take up a lot of space. -arrow and line diagrams take up a lot of space and make it difficult to count electrons. -written configurations make it easy to lose count of electrons and do not show the distribution of electrons in orbitals.
Answers: 3

Chemistry, 22.06.2019 14:30
1) describe the physical layout of the ocean floor ? 2) explain how the dumbo octopus swims differently than other octopus species and why this would be an advantage in the aphonic zone . 3) why are the types of organisms that live at each underwater hot vent so dramatically different ?
Answers: 3

Chemistry, 22.06.2019 19:40
Scientists have developed an explanation of a phenomenon from several verified hypotheses. the explanation has been confirmed through numerous experimental tests.which option best describes this explanation? a. scientific lawb. research questionc. hypothesisd. scientific theory
Answers: 3

Chemistry, 22.06.2019 20:00
What happens to the temperature of a substance when the average kinetic energy of its particles increases?
Answers: 3
You know the right answer?
Using the balanced equation below,
how many grams of manganese(III)
oxide would be produced f...
oxide would be produced f...
Questions

















Health, 22.11.2019 23:31



Mathematics, 22.11.2019 23:31

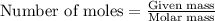 ......(1)
......(1)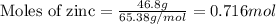
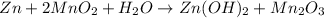
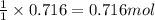 of manganese(III) oxide
of manganese(III) oxide