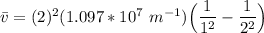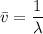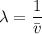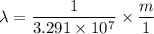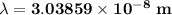Chemistry, 08.07.2021 15:50 lalkjlkeu2409
The energy levels of hydrogenlike one-electron ions of atomic number Z differ from those of hydrogen by a factor of Z^2. Predict the wavelength of the 2s--->1s transition in He+.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:30
Asample of air with a volume of 2.20m3 at a pressure of 105 kpa and a temperature of 30c is cooled to 10c and the pressure is reduced to 75.0 kpa. what is the new volume? 6.9 1.34 2.56 43.0 2.88
Answers: 1

Chemistry, 22.06.2019 13:00
What is the mass of 2.00 l of an intravenous glucose solution with a density of 1.15 g/ml?
Answers: 2

Chemistry, 22.06.2019 14:20
Which statement explains why the bonds between non metals tend to be covalent? the bonds are found to be nondirectional they have large differences in electronegativity they have small differences in electronegativity they have ions that produce an electrostatic pull
Answers: 1

Chemistry, 22.06.2019 15:00
What is the most important factor in determining climates.
Answers: 1
You know the right answer?
The energy levels of hydrogenlike one-electron ions of atomic number Z differ from those of hydrogen...
Questions











Computers and Technology, 15.11.2019 22:31









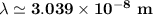
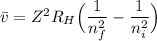
 = ???
= ???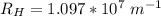
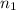 = 2
= 2