
Chemistry, 02.10.2021 22:10 alizeleach0123
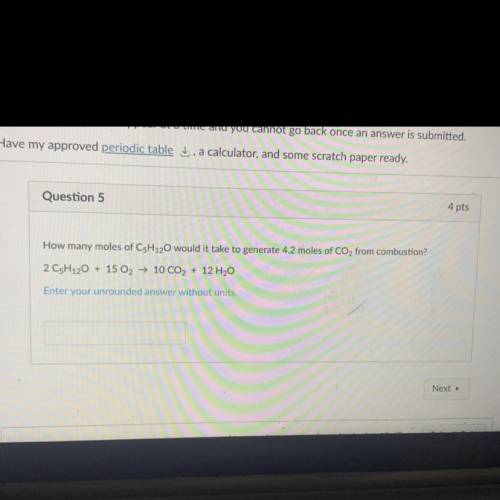
How many moles of C5H120 would it take to generate 4.2 moles of CO2 from combustion?
2 C5H120 + 15 O2 → 10 CO2 + 12 H20
Enter your unrounded answer without units.
Next →

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:30
18. use the activity series to predict whether the following synthesis reaction will occur. write the chemical equations for the reaction if it's predicted to occur. (s) + o2(g) -> *note: it is possible.*
Answers: 1

Chemistry, 22.06.2019 03:00
Explain how the integumentary system plays a crucial role in the ability to maintain homeoestasis
Answers: 1

Chemistry, 22.06.2019 05:40
Why did southern business leaders want to increase the number of slaves
Answers: 1

Chemistry, 22.06.2019 07:30
The scheme below is from a series of reactions that are part of a synthesis of vitamin a. answer the following questions with reference to this scheme. (i) what is "reagent a"? (ii) draw a step-by-step mechanism which explains the formation of compound c from compound b (iii) which reagents would you use to form compound e from compounds c and d (reagents b and c)? for each reagent suggested above in (ii) explain the role of the reagent in the reaction to (iv) form compound e. you may wish to do this by drawing a mechanism. 1. addition of reagent a но reagent a 2. н,о" thо oh нон-с compound a. compound b. compound c .ch-оh 1. reagent b "сно 2. reagent c сh oh compound e. compound d.
Answers: 2
You know the right answer?
How many moles of C5H120 would it take to generate 4.2 moles of CO2 from combustion?
2 C5H120 + 15...
Questions








History, 23.10.2019 19:30

History, 23.10.2019 19:30








Mathematics, 23.10.2019 19:30





