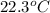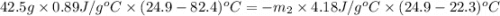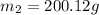Physics, 29.07.2019 02:00 christabell0303
A42.5 g piece of aluminum (which has a molar heat capacity of 24.03 j/ocmol) is heated to 82.4oc and dropped into a calorimeter containing water (specific heat capacity of water is 4.18 j/goc) initially at 22.3oc. the final temperature of the water is 24.9oc. calculate the mass of water in the calorimeter. ignore significant figures for this problem.

Answers: 1


Another question on Physics


Physics, 21.06.2019 23:30
The velocity of car is 80.0 miles per hour. what is the velocity of car in meters per second? (1 km -0.621 miles) m/s
Answers: 2

Physics, 22.06.2019 00:30
Occurs when an energy source transfers heat directly to another subject space; an example would be an object becoming warm by sitting in the sunshine
Answers: 1

Physics, 22.06.2019 15:50
If the work required to stretch a spring 3 ft beyond its natural length is 15 ft-lb, how much work is needed to stretch it 27 in. beyond its natural length?
Answers: 1
You know the right answer?
A42.5 g piece of aluminum (which has a molar heat capacity of 24.03 j/ocmol) is heated to 82.4oc and...
Questions





Mathematics, 08.08.2019 20:20

Mathematics, 08.08.2019 20:20

Chemistry, 08.08.2019 20:20













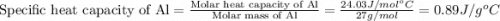
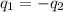
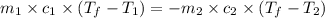
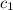 = specific heat of aluminum =
= specific heat of aluminum = 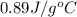
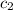 = specific heat of water =
= specific heat of water = 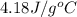
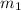 = mass of Al = 42.5 g
= mass of Al = 42.5 g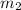 = mass of water = ?
= mass of water = ?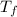 = final temperature of water =
= final temperature of water = 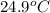
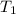 = initial temperature of Al =
= initial temperature of Al = 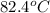
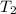 = initial temperature of water =
= initial temperature of water =